Free Sales Certificate / Certificate of Free Sale / Certificate of Marketability - EU / UK / Switzerland
Applying for Free Sales Certificate (FSC) / Certificate of Free Sale (CFS) / Certificate of Marketability for non-European countries.
Are you a Medical Device or In Vitro Diagnostic Device manufacturer aiming to conquer international markets?
Why You Need an FSC?
With the CE marking in place your products can be legally marketed in Europe. The CE marking is an indication to show conformity to all obligations for medical devices, as required by the Medical Device Regulations.
However, for entering certain non-European markets a FSC must be provided by the manufacturer. It demonstrates, from the government point of view, that you place your products legally, with the CE mark, on the European market.
The FSC is issued by the Competent Authority of the country where the manufacturer or his EC REP / UKRP / CH-REP has his registered place of business. If the manufacturer is not situated in Europe, only the EC REP / UKRP / CH-REP is able to obtain the FSC on behalf of the manufacturer.
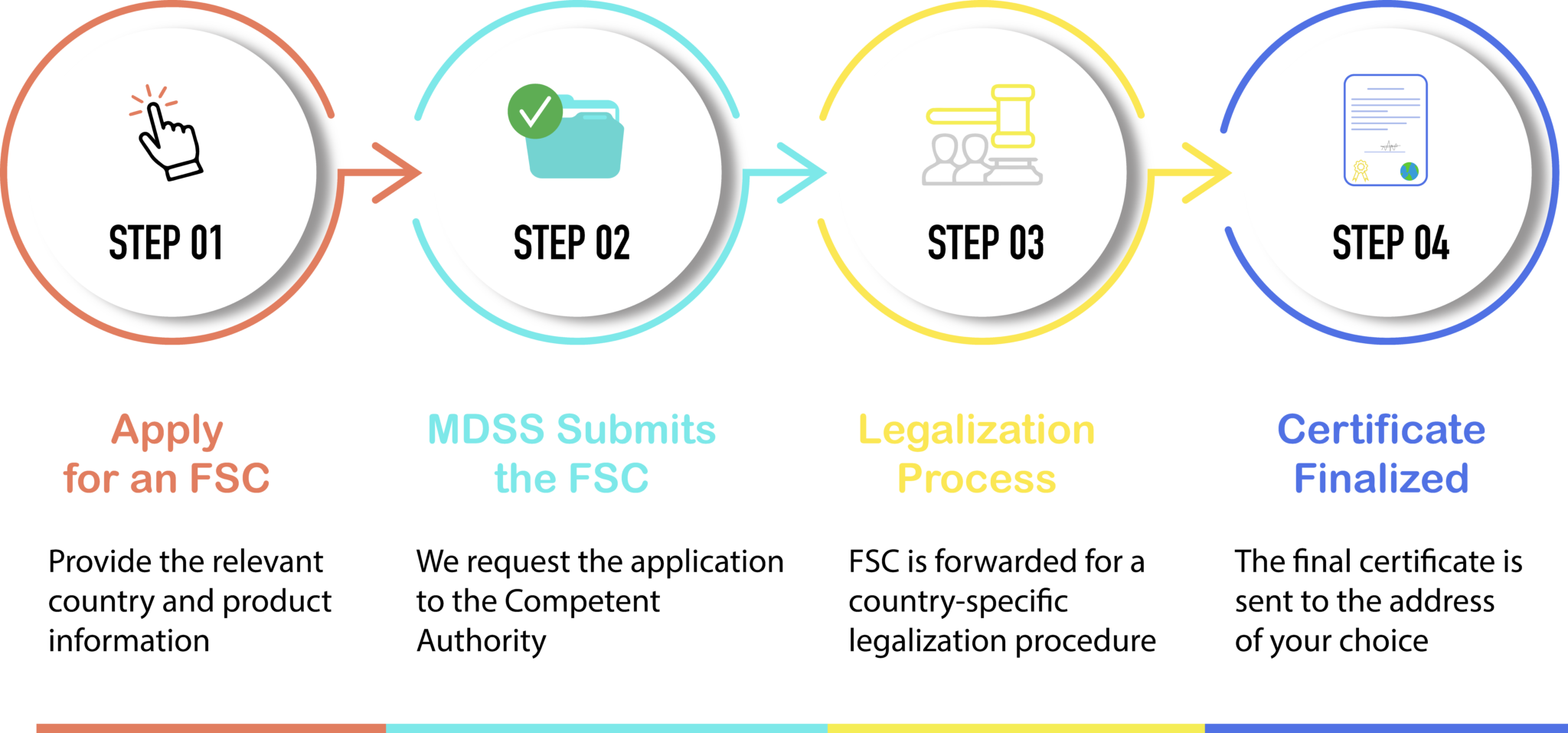
MDSS currently implemented a fast process for over 60 countries:
Algeria, Argentina, Albania, Bahrain, Bangladesh, Belarus, Bolivia, Bosnia and Herzegovina, Brazil, Chile, Colombia, Costa Rica, Dominican Republic, Ecuador, Egypt, El Salvador, Ethiopia, Georgia, Guatemala, Honduras, Hong Kong, India, Indonesia, Iran, Iraq, Israel, Jamaica, Japan, Jordan, Kazakhstan, Kuwait, Kyrgyzstan, Malaysia, Mexico, Montenegro, Myanmar, Nicaragua, Nigeria, North Macedonia, Oman, Pakistan, Panama, Paraguay, Peru, Philippines, Qatar, Russia, Saudi Arabia, Serbia, Singapore, South Africa, South Korea, Sri Lanka, Taiwan, Thailand, Tunisia, Turkey, Ukraine, United Arab Emirates, Uruguay, Venezuela, Vietnam.
If you would like to apply for a FSC for a country not listed above let us know and we will find out the requirements for it.
The identification of the country is crucial because, once the FSC has been issued by the Authority, different requirements are imposed by the country in regards to the legalization (apostille, translation, authentication by the consulate etc.).

Why Choose MDSS?
- Expertise You Can Trust: With years of experience in regulatory affairs, we know the ins and outs of international market access.
- Streamlined Process: We navigate the complexities, ensuring a smooth and efficient application for your FSC.
- Global Reach: No matter where you’re based, MDSS facilitates market entry, enabling you to reach patients worldwide.
Process for obtaining Free Sales Certificate